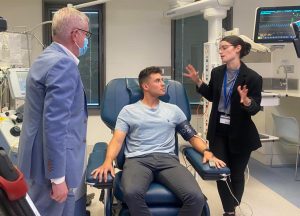
A research program looking into more effective treatment for those who have heart problems linked to type 2 diabetes is looking for participants.
The SURPASS-CVOT study is testing the efficacy of an experimental drug, tirzepatide, in preventing cardiovascular events induced by type 2 diabetes.
Funded by Eli Lilly Australia, the clinical trial has been underway at the Royal Adelaide Hospital to drive research-informed decisions and to deliver real health outcomes for the community.
During Heart Research Month, the study would like to enrol more suitable candidates, who are people with type 2 diabetes, who are at least 40 years old and with certain types of cardiovascular disease.
“Cardiovascular disease continues to be the leading cause of death in patients with type 2 diabetes. The prevention of cardiovascular complications and the cardiovascular safety of treatments should be a primary objective when selecting treatment,” Research Manager Denise Healy said.
“All the participants so far are very pleased with the effect of the medication on their diabetes, heart disease and weight loss.”
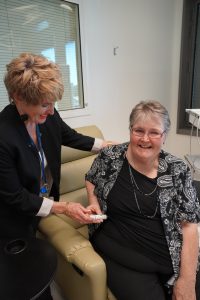
Research Manager Denise Healy and participant Rosemary Barraclough.
Up to 12,500 participants worldwide, including 400 in Australia, will be taking part in this study.
Participant Rosemary Jones-Anspach has noticed weight loss and found herself feeling satiated with smaller portions of food without feeling as if she is depriving herself.
“I don’t crave sugar as much,” she said.
Participant Rosemary Barraclough said not only has she lost a lot of weight but her mood has improved. She also finds herself more active and able to walk easily, not needing to rely on a wheelchair anymore.
“I’m starting to see the rewards – both physically and mentally. For the first time I am confident to engage with people I may not know very well,” she said.
She said the monthly visits to hospital have become a “social event” for her.
The trial is in Phase 3 and is expected to be completed in 2024.
Interested participants will undergo a pre-screening process before joining the research.
To find out more about the study or to join, potential candidates should contact Ms Denise Healy on 0409 877 807 for a confidential discussion.