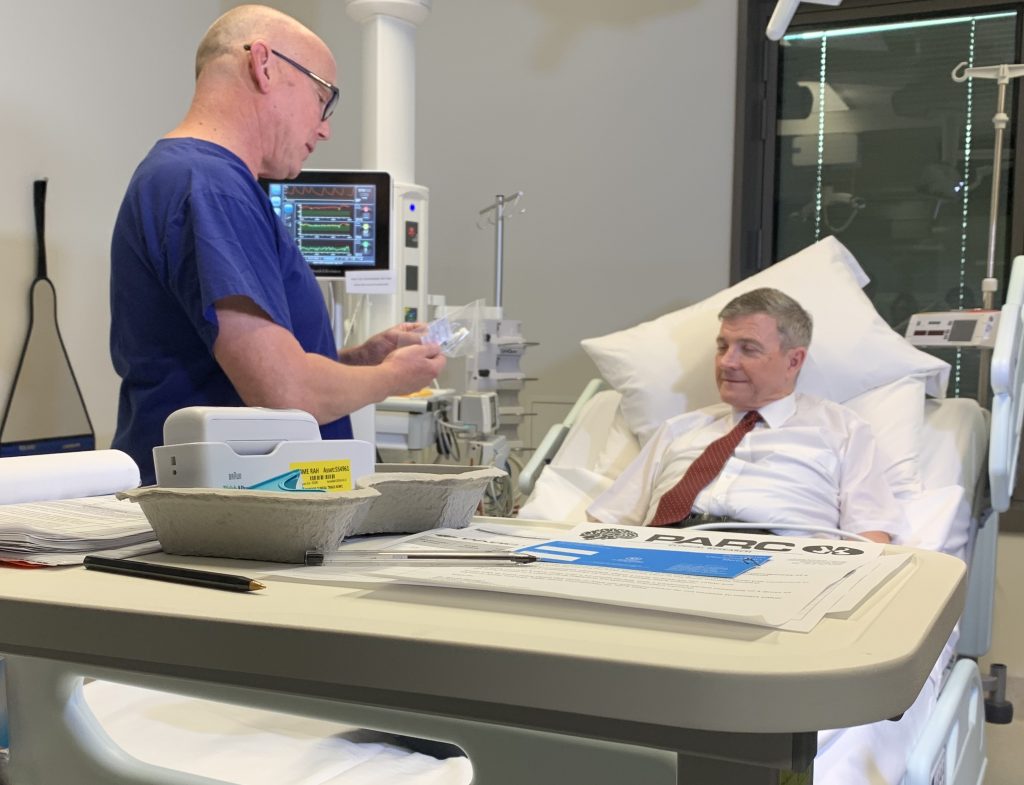
In a national breakthrough, the first human trials of a COVID-19 vaccine developed in Australia have begun at PARC Clinical Research at our Royal Adelaide Hospital.
This is a significant development in the fight against COVID-19 and the vaccine and trial program, developed in South Australia, could result in an historic medical breakthrough.
The first stage of the trial will involve 40 pre-screened candidates receiving two doses of the vaccine three weeks apart.
The development of antibodies and other immune responses will be determined two weeks after the second injection, so the results of the first trial will available in around two months’ time.
Principal Investigator Professor David Gordon, a Microbiologist and Infectious Diseases Physician with SA Pathology, said the trial is the first of several planned and involves testing the safety and immune responses to the vaccine.
“This COVID-19 vaccine has already shown promising results in animal models, so it is exciting to take this national breakthrough to human trials in Adelaide,” Dr Gordon said.
Central Adelaide Local Health Network Clinical Immunologist, Dr Pravin Hissaria, said the trial participants’ involvement was a vital component of the medical research to find a vaccine.
“Thanks to actions of all South Australians, we have managed to limit the effects of COVID-19 on our community,” Dr Hissaria said.
“Now, members of the public have stood up to be amongst the first people in the world to receive a trial vaccine. Their actions could help us find a permanent solution to this devastating global pandemic.”
For more information about participating in the trial, call PARC Clinical Research at the Royal Adelaide Hospital on 08 7074 2996.
*Pictured are PARC Clinical Research Director, Prof Guy Ludbrook, with the first trial participant Ian Tindall.